Abstract
Introduction / Aims CD19 CAR-T therapy for relapsed/refractory (r/r) large B-cell lymphoma (LBCL) has been implemented by the UK National Health Service since December 2018. First data from the UK real-world experience suggested inferior outcomes compared to the pivotal trials, but results have improved over time. We aimed to identify potential differences in patient selection and management between treatment eras, reflecting the CAR-T learning curve, as well as determinants of improved outcome.
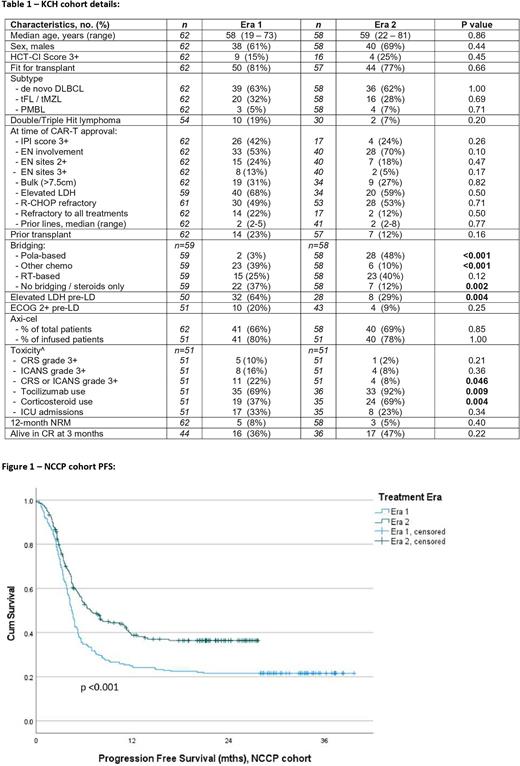
Methods We included 120 consecutive LBCL patients approved for treatment with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) at King's College Hospital London (KCH cohort) and split them into 2 treatment eras - Era 1 (Dec 2018 - Dec 2019), and Era 2 (Jan 2020 - Mar 2022). Survival analyses were extended to the UK national cohort of 446 patients intended to be treated (ITT) with CAR-T across 10 UK CAR-T centers, with a median follow-up of 25.7 months.
Results In the KCH cohort, baseline patient and disease characteristics did not significantly differ between Era 1 and 2, except for a lower incidence of patients with pre-lymphodepletion (LD) elevated LDH (eLDH) in Era 2 (see Table). CAR-T product choice and median vein-to-vein time were similar in both eras. Bridging strategies evolved with more use of polatuzumab vedotin-based bridging in Era 2 (48% vs 3%, p<0.001) as well as a trend to more radiotherapy bridging (40% vs 25%, p=0.12). There were fewer bridging failures (drop-out after leukapheresis due to clinical deterioration or death) in Era 2 vs 1: 3% vs 14%, p=0.05. In total, 88% (Era 2) vs 82% (Era 1) of the ITT population proceeded with CAR-T infusion (p=0.45).
When assessing CAR-T toxicity in Era 2 vs Era 1, the incidence of high-grade toxicity was lower (Grade 3+ CRS/ICANS 8% vs 22%, p=0.046), with more liberal use of tocilizumab and corticosteroids in Era 2 (see Table). One-year non-relapse mortality (NRM) was similar between eras (5% vs 8%, p=0.40). Progression-free survival (PFS) showed a trend to improvement in Era 2 (median PFS 11.3mths vs 5.2mths, 1-yr-PFS 41% vs 31%, p=0.057) and overall survival was significantly longer (median OS NR vs 11.7mths, 1-yr-OS 71% vs 48%, p=0.006).
We extended our survival analysis to the UK-wide CAR-T cohort of 446 patients. Similarly, for Era 2 vs 1, we observed a lower incidence of eLDH pre-LD (50% vs 66%, p 0.004), increased use of polatuzumab- based bridging (45% vs 3%, p<0.001), fewer bridging failures (10% vs 20%, p=0.003), and a higher use of tocilizumab/steroids (77%/49% vs 60%/34% (both p<0.001). In addition, patients in Era 2 were older (median 62y vs 58y, p<0.001), had better pre-LD ECOG PS (7.5% vs 12% ECOG 2+, p=0.002) and were more likely to receive axi-cel (78% vs 69%, p=0.004). Outcome analyses of the total cohort showed unequivocally that both PFS and OS were longer in the more recent era (median PFS 6.8mths vs 4.4mths, 1-yr-PFS 39% vs 25%, p<0.001; median OS NR vs 8.3mths, 1-yr-OS 59% vs 40%, p<0.001).
In univariate analysis of both PFS and OS including all patients, baseline IPI, 3+ extranodal sites, baseline eLDH, bulky disease, refractoriness to all prior therapy, lack of response to bridging, pre-LD eLDH, pre-LD ECOG 2+, pre-LD CRP, tisa-cel use and Treatment Era 1 vs 2 were significantly associated with inferior survival. On MVA, 3+ extranodal sites (HR 2.0 [1.1 - 3.7]); pre-LD eLDH (HR 1.7 [1.1 - 2.8]) and lack of bridging response (HR 1.6 [1.0 - 2.6]) remained significant for PFS, and 3+ EN sites (HR 2.5 [1.3 - 4.8]); pre-LD eLDH (HR 2.8 [1.5 - 4.9]); ECOG 2+ (HR 2.0 [1.0 - 4.0]) and lack of bridging response (HR 2.1 [1.2 - 3.7]) for OS.
Conclusions Our data show improvement over time in survival outcomes for LBCL patients treated with CD19 CAR-T at our center and nationally which appear to be mainly driven by improvements in bridging therapy. Incidence of high-grade CAR-T toxicities was low and seems to have further reduced with more proactive toxicity management in the recent era.
Disclosures
Sanderson:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel to educational meetings, Speakers Bureau; Kite Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel to educational meetings, Speakers Bureau. O'Reilly:Kite Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel to educational meetings, Speakers Bureau; Novartis: Honoraria, Other: travel to educational meetings. Bloor:Janssen: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau. Chaganti:Roche: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; Orion Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead/Kite: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Atara Biotherapeutics: Consultancy, Honoraria; Adicet Bio: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Latif:Astellas: Honoraria; Abbvie: Honoraria; Kite: Honoraria; Novartis: Honoraria; Jazz: Honoraria; Takeda UK: Honoraria. Benjamin:Bristol Myers Squibb/Celgene: Research Funding; Amgen: Research Funding. Patten:Abbvie: Honoraria; AstraZeneca: Honoraria; Beigene: Honoraria; Gilead Sciences: Honoraria, Research Funding; Janssen: Honoraria; Roche: Research Funding. Yallop:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Other: Meeting support, Research Funding; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Other: Meeting Support. Roddie:Kite Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kuhnl:BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Kite: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal